Scientific Breakthrough: New Non-Transition Metal Catalyst Enables Ammonia Synthesis at Low Temperature and Pressure
Background: A New Challenge in Ammonia Synthesis
Ammonia, essential for fertilizer production, is also a potential hydrogen carrier for renewable energy infrastructure. However, traditional ammonia synthesis relies on high temperatures (400-500°C), high pressures (10-30 MPa), and transition metal catalysts such as iron or ruthenium, making it one of the most energy-intensive industrial processes, accounting for over 1% of global energy consumption. To reduce this energy demand, scientists have been exploring new catalysts capable of efficient operation under lower temperature and pressure conditions.
Research Highlights: Activating Nitrogen and Synthesizing Ammonia with Non-Transition Metal Catalyst
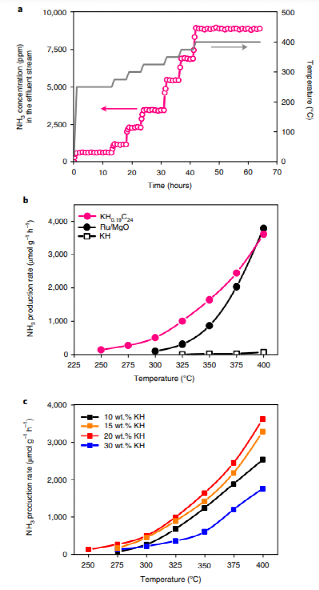
The study introduces a groundbreaking non-transition metal catalyst, potassium hydride-intercalated graphite (KH0.19C24), which successfully activates nitrogen at ambient pressure and produces ammonia under relatively mild conditions (250-400°C, 1 MPa). This novel catalyst achieves ammonia synthesis rates comparable to the classical Ru/MgO catalyst, even exceeding Ru in production rates by 1-10 times at 300°C. With a 20 wt% loading, KH0.19C24 demonstrated optimal catalytic activity under these conditions.
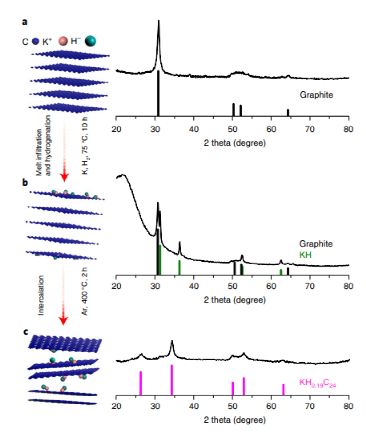
Catalyst Preparation and Mechanistic Insights
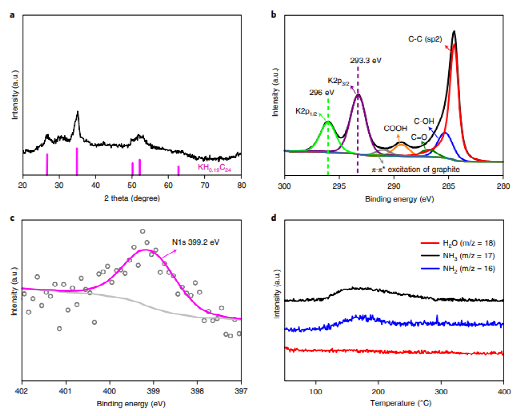
The KH0.19C24 catalyst is synthesized by intercalating potassium hydride within graphene layers, resulting in a nanoscale dispersion in a carbon matrix. Experiments and computational models reveal that this structure is crucial for nitrogen activation, with hydrogen atoms in the catalyst playing a vital role in the hydrogenation steps leading to ammonia formation. Detailed structural analysis confirms the stable intercalation and dispersion of potassium hydride in the graphite, which supports high catalytic performance.
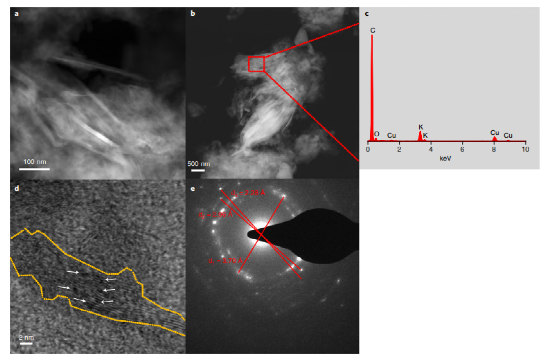
New Mechanistic Insights: Ammonia Formation on the Catalyst Surface
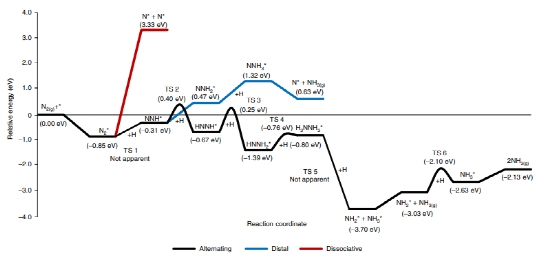
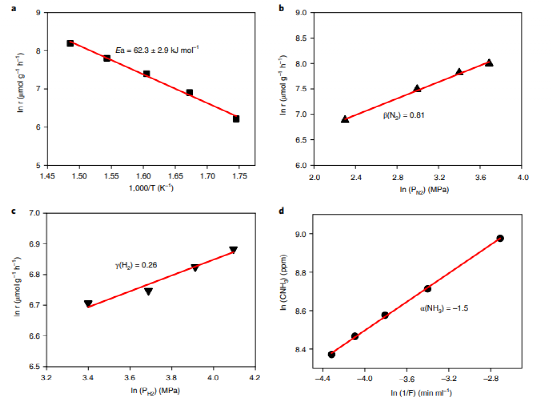
The study further investigates the kinetics and reaction mechanisms of ammonia synthesis on KH0.19C24. Kinetic analysis shows an activation energy of just 62.3 kJ mol-1, significantly lower than traditional transition metal catalysts. Findings indicate that nitrogen activation and dissociation on the catalyst surface is a key rate-determining step, effectively addressed by this catalyst. Density functional theory (DFT) calculations show that a stepwise hydrogenation mechanism is energetically favorable, markedly reducing the energy requirements for ammonia synthesis.
Future Outlook: A Green Revolution in Ammonia Synthesis Technology
This study introduces a promising low-cost, low-energy pathway for ammonia synthesis, offering a transformative solution for green development in the fertilizer and energy sectors. The advent of KH0.19C24 may drive a shift towards sustainable ammonia synthesis, supporting ammonia's potential as a clean energy carrier and potentially sparking a revolutionary advancement in synthesis technology.