Advancements in Secondary Reforming Catalyst Technology for Ammonia Synthesis
Advancements in Secondary Reforming Catalyst Technology for Ammonia Synthesis
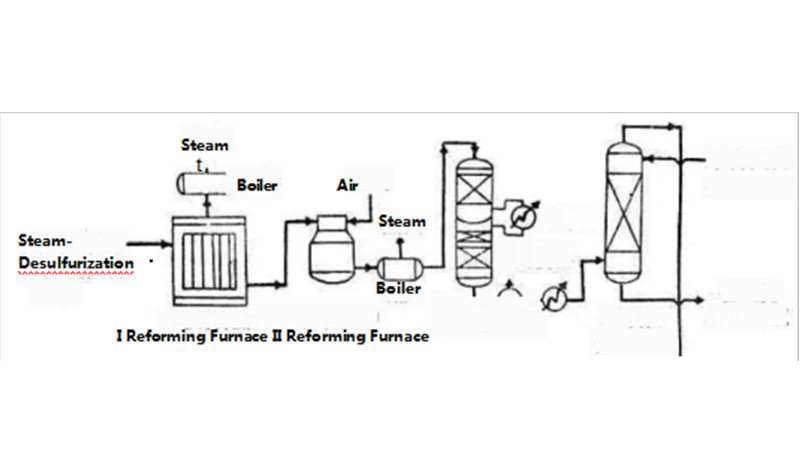
In the synthesis of ammonia, there often exists unconverted methane gas during the primary steam reforming process. Therefore, in the secondary steam reforming process, air is introduced as an oxidant to selectively oxidize this portion of methane using catalysts, thereby removing it and obtaining a large amount of nitrogen component.
Optimizing Methane Conversion
This method can reduce the load on the primary steam reforming process, saving the amount of nickel metal used in the primary steam reforming, and thus increasing the overall efficiency of the catalyst. Additionally, the high-temperature gas at the outlet of the secondary steam reforming furnace can provide the heat required for primary steam reforming furnace, significantly increasing the heat usage of the natural gas feedstock and maximizing energy utilization.
Selecting Catalysts for Secodary Steam Reforming
The reactions involved in the secondary steam reforming include the partial oxidation reaction of methane. However, due to the involvement of side reactions such as hydrogen and carbon monoxide oxidation, it is necessary to introduce catalysts into this process. Palladium-based catalysts are the optimal catalysts for promoting methane combustion. However, in industry, nickel-based steam reforming catalysts are generally used due to their effectiveness.
Industrial Implementation of Secondary Steam Reforming
The primary steam reforming in ammonia synthesis plant of China National Offshore Oil Corporation (CNOOC) was fully introduced and commissioned by Chiyoda Corporation, with a designed annual output of 300,000 tons of ammonia, using natural gas as feedstock and employing the ICI-AMV ammonia synthesis technology from the UK. The secondary steam reforming furnace adopts a vertical shaft furnace, with 20% excess air added, aiming to shift the load of primary steam reforming furnace backward, achieving energy savings and equipment investment reduction.
Research in Fixed-Bed Reactors
Currently, research work mainly takes place in externally heated fixed-bed reactors. Due to the exothermic nature of the reaction, "hot spots" are easily generated, leading to catalyst deactivation. Choudhary et al. investigated the reaction results of methane partial oxidation to synthesis gas using Ni and/or Co catalysts supported on different metal oxides (ZrO2, ThO2, UO2, TiO2, SiO2) under high space velocities (GHSV = 5.2 × 105 h-1). Ni/ZrO2, Ni/ThO2, and Ni/UO2 exhibited good catalytic activity for methane partial oxidation, with the activity order as follows: Ni/ThO2 > Ni/UO2 > Ni/ZrO2. Yu et al. examined the methane partial oxidation activity and selectivity of four catalysts with a Ni loading of 8 wt.%: Ni/γ-Al2O3, Ni/δ-Al2O3, Ni/θ-Al2O3, and Ni/α-Al2O3. The results showed that at 1043 K, the methane conversion rate and selectivity for CO and H2 were ranked as follows: Ni/γ-Al2O3 < Ni/δ-Al2O3 < Ni/θ-Al2O3 ≈ Ni/α-Al2O3.

Advancements with SYAMCAT Steam Reforming Catalyst
In addition to these advancements, SYAMCAT steam reforming catalyst plays a crucial role in optimizing the efficiency and performance of the reforming process. With its advanced formulation and superior catalytic properties, SYAMCAT offers significant advantages in terms of activity, stability, and longevity.
Contact Us for SYAMCAT Solutions
For further inquiries about SYAMCAT steam reforming catalyst or to discuss how it can benefit your specific operations, please feel free to contact us. Discover the difference SYAMCAT can make in optimizing your steam reforming processes and achieving your operational goals. We look forward to hearing from you!

